Solved Show that the compressibility factor of van der Waals

Answer to Solved Show that the compressibility factor of van der Waals

Physical Chemistry The Compression Factor (Z) [w/1 example]

Van der Waals Equation:Calculate the expansion and compresibility coefficient

6.3: Van der Waals and Other Gases - Physics LibreTexts
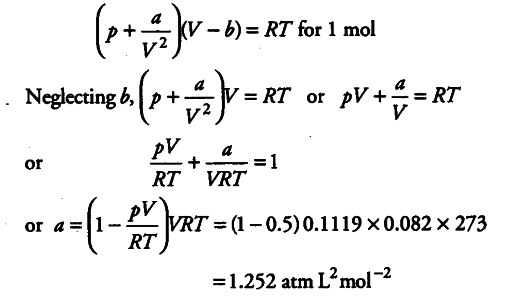
The compression factor (compressibility factor) for one mole of a van - CBSE Class 11 Chemistry - Learn CBSE Forum
How I find the a and b constant in the Van der Waals equation? - Quora

Van Der Waals Equation - an overview
upload.wikimedia.org/wikipedia/commons/thumb/6/6e/
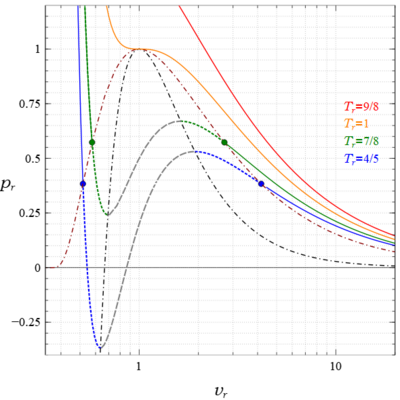
Thermo] Derivation of compressibility factor vs reduced pressure

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

P=V−bRT−D2a where a=64Pc27R2Tc2 and b=8PcRTc The van
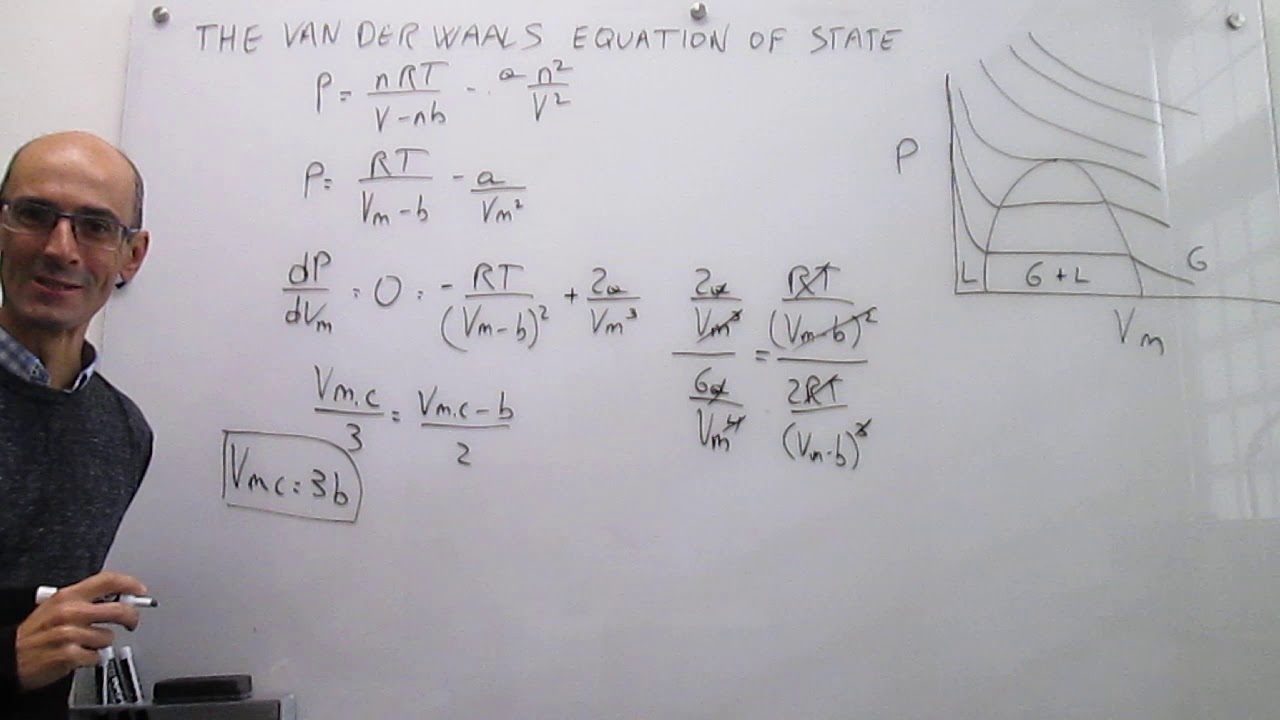
The van der Waals equation of state at the critical point

Solved We showed, for a van der Waals gas, that the

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to

ReasonThe kinetic theory postulates of negligible volume of gaseous molecules and intermolecular forces of attraction do not stand correct high pressure and low temperature.AssertionVan der Waals equation describes the behaviour of real











